Home | N,N-Dimethylformamide (DMF) is not a solvent above suspicion
N,N-Dimethylformamide (DMF) is not a solvent above suspicion
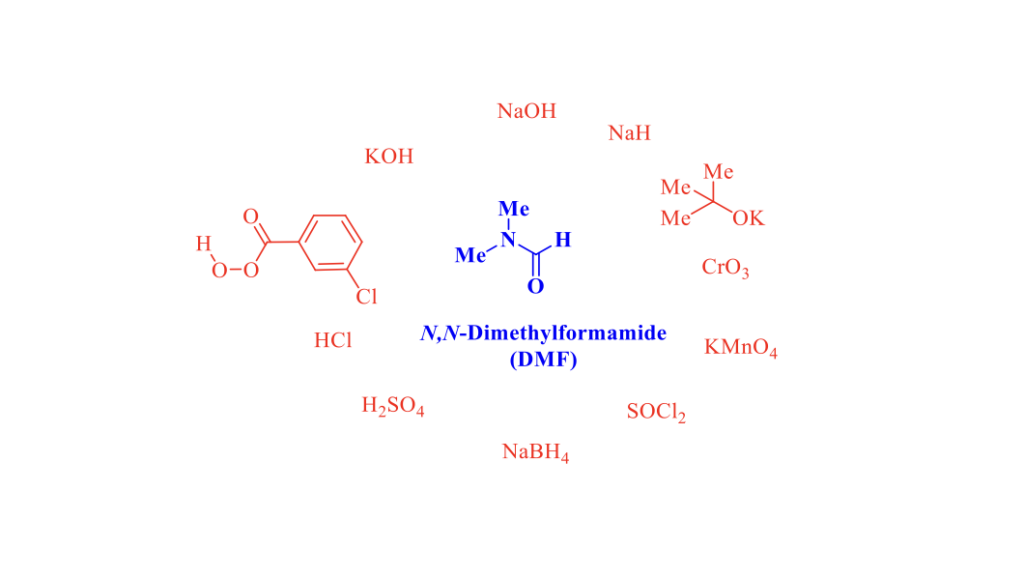
N,N-Dimethylformamide (DMF) is an aprotic polar solvent very popular in organic synthesis. In many transformations, DMF is also a versatile reagent, the most commonly used being the Vilsmeier reagent. [1] In 2020, Q. Yang and Coll., from Corteva Agriscience (Indianapolis, Indiana, USA) published a very interesting and complete review, with an extensive bibliography, on the potential risks associated with the use of DMF as a solvent. [2] Numerous reports by industrial and academic researchers have clearly shown that DMF is not an inert solvent with respect to bases (NaH, KOH), acids (HCl, H2SO4), oxidizing reagents (CrO3, KMnO4, mCPBA) and reducing reagents (NaBH4). This article is particularly instructive.
[1] For an excellent recent review, see: J. Muzart, Molecules 2021, 26, 6374. https://doi.org/10.3390/molecules26216374S.
[2] Q. Yang, M. Sheng, Y. Huang, Org. Process Res. Dev. 2020, 24, 1586-1601.
