Home | Palladium-catalyzed cyanation of aryl sulfonium salts with potassium ferrocyanide
Palladium-catalyzed cyanation of aryl sulfonium salts with potassium ferrocyanide
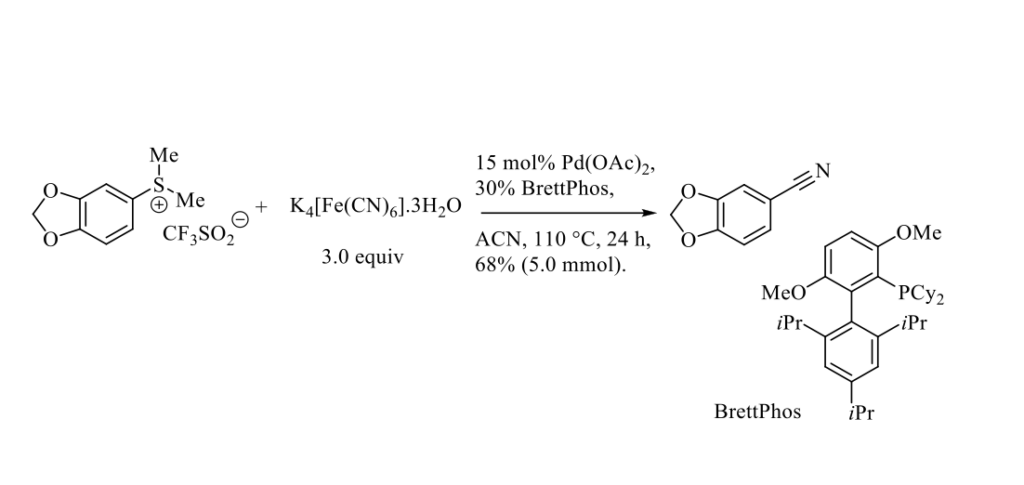
Aromatic nitriles are of significant importance in synthetic organic chemistry, as they are crucial moieties in many drugs, pesticides, herbicides and natural products, as well as in dyes and pigments. In addition, the nitrile function can be easily converted into various other functions such as amine, acid, aldehyde, in particular. Palladium-catalyzed cross-coupling reactions between aromatic halides or equivalents and different cyanide sources has been widely used to prepare aromatic nitriles. [1] Very recently, Cao and Coll. reported a palladium-catalyzed cyanation of aryl dimethylsulfonium salts (mainly triflate) using potassium ferrocyanide (K4[Fe(CN)6]) as safe and nontoxic (in rats, oral LD50 is 1.6 to 3.2 g/kg body weight) cyanide source. [2] A representative example is presented in the Scheme below.
The bulky monodentate phosphine ligand BrettPhos proved to be the most efficient ligand. It should be pointed out that the aryl dimethylsulfonium salts were prepared from their corresponding thiomethylether, excess of iodomethane and stochiometric amount of silver triflate salts (500 €/100 g). A full mechanistic investigation of this palladium-catalyzed cyanation is reported.
[1] M. Neetha, C. M. A. Afsina, T. Aneeja, G. Anilkumar, RSC Adv. 2020, 10, 33683-33699.
[2] M. Liu, B. Cui, C. Zhong, Y. Shi, Y. Dang, C. Cao, Org. Lett. 2023, 25, 3989-3994.
