Home | Palladium-catalyzed Buchwald-Hartwig amination
Palladium-catalyzed Buchwald-Hartwig amination
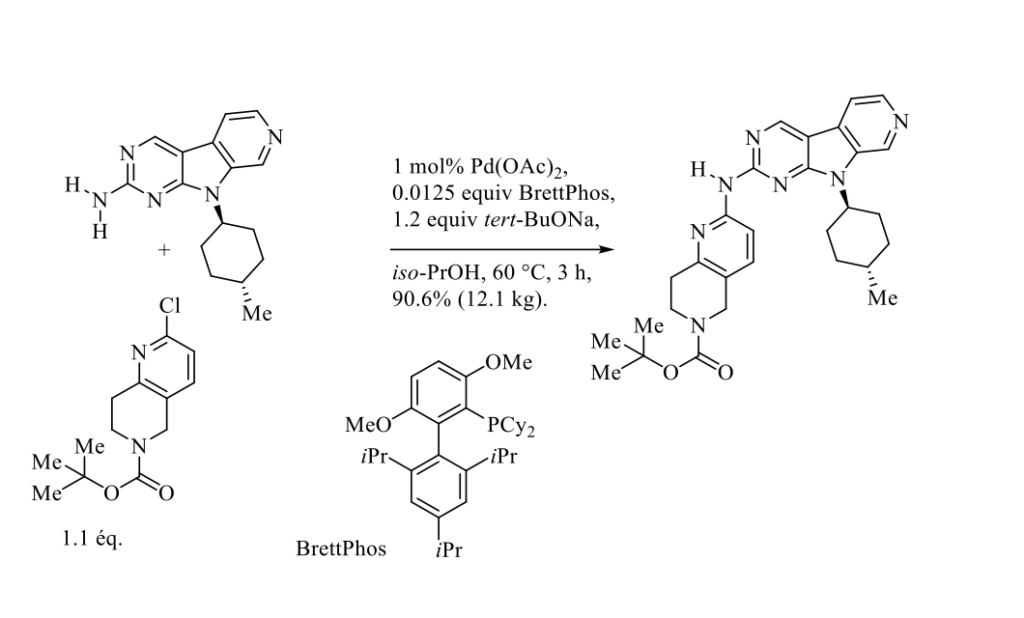
Since the pioneering investigations of Migita et Coll. in 1983 and the simultaneous outstanding contributions of Buchwald and Hartwig over many years, the palladium-catalyzed cross-coupling of NH-containing substrates and heteroaryl halides and pseudohalides has emerged as most powerful and useful tool for the formation of C(sp2)-N bonds. [1] This methodology has found numerous applications in the preparation of pharmaceutical ingredients, from laboratory scale to large-scale production. [2] For example, during the course of the development of multi-kilogram-scale synthetic route to the drug candidate AMG 925 to treat acute myelogenous leukemia, a palladium-catalyzed Buchwald-Hartwig amination was optimized to provide an advanced intermediate in excellent yield, as reported in Scheme below. [3]
It should be pointed out that all attempts to carry out this transformation, via a nucleophilic aromatic substitution, by treatment of starting materials with strong bases such as KHMDS, NaHMDS, LiHMDS, tert-BuOK, tert-BuONa, tert-BuOLi in N-Methyl-2-pyrrolidone at 100 °C failed, as very low yields (<10%) of desired adduct were observed.
[1] R. Dorel, C. P. Grugel, A. M. Haydl, Angew. Chem. Int. Ed. 2019, 58, 17118-17129 and cited literature.
[2] P. A. Forero-Cortés, A. M. Haydl, Org. Process Res. Dev. 2019, 23, 1478-1483 and cited literature.
[3] C. Affouard, R. D. Crockett, K. Diker, R. P. Farrell, G. Gorins, J. R. Huckins, S. Caille, Org. Process Res. Dev. 2015, 19, 476-485.
