Home | Deoxyfluorination of electron-deficient phenols with 2-chloroimidazolium dihydrogen trifluoride salt.
Deoxyfluorination of electron-deficient phenols with 2-chloroimidazolium dihydrogen trifluoride salt.
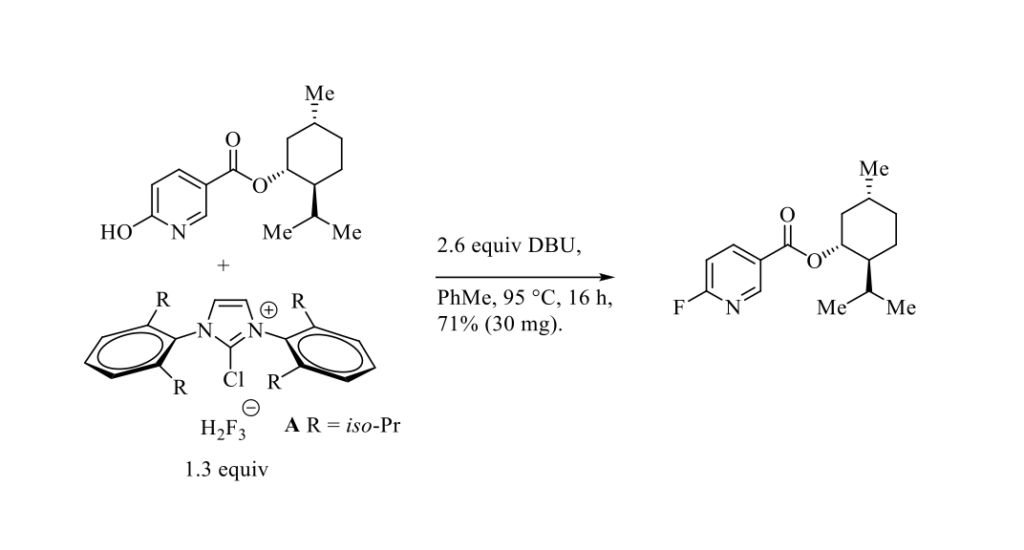
An efficient preparation of 2-chloro-1,3-bis(2,6-diisopropylphenyl)imidazolium dihydrogen trifluoride salt A (Scheme below ) in aqueous media under ambient conditions, using hypochlorite as a chlorinating agent, has been reported by Jelen and Tavčar in 4 steps from glyoxal and 2,6-diisopropylaniline in 62% overall yield on 10 g scale. [1] This air-stable and moisture-insensitive deoxyfluorination reagent A has been successfully used to convert various electron-deficient phenols (23 examples) into their corresponding aryl fluorides in the presence of DBU, as exemplified in the Scheme below.
[1] J. Jelen, G. Tavčar, Org. Lett. 2023, 25, 3649-3653.
