Home | An efficient gram scale synthesis of (±)-Sparteine
An efficient gram scale synthesis of (±)-Sparteine
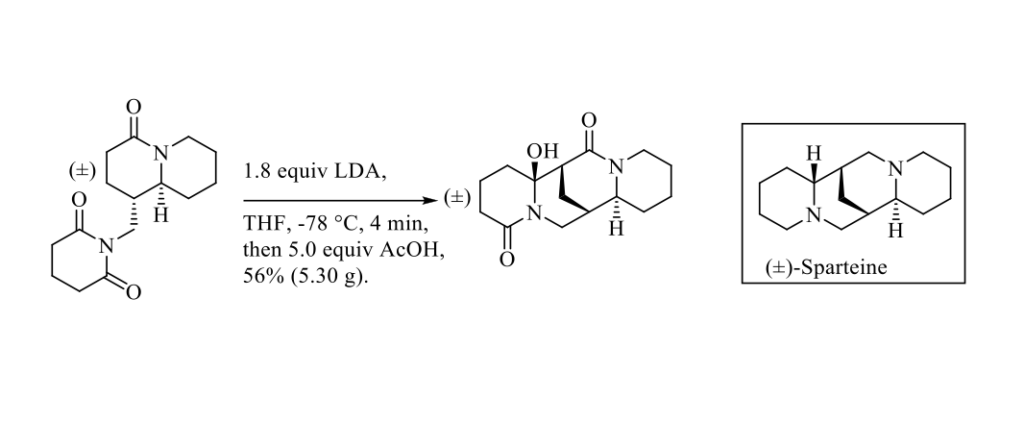
(-)-Sparteine first isolated from Lupinus Barbiger in 1932 and its (+)-Sparteine surrogate prepared from (-)-Cytisine, another alkaloid easily isolated from Laburnum anagyroides seeds, have been widely used as chiral ligands in asymmetric synthesis. [1] Very recently, S. E. Reisman, and Coll. published an efficient seven-step gram scale synthesis of (±)-Sparteine, based on intramolecular enolate addition to the glutarimide moiety to construct the sparteine skeleton, as outlined in the Scheme below. [2]
It should be pointed out that a ten-step gram-scale synthesis of (-)-Sparteine has been reported. [3]
[1] M. J. Dearden, C. R. Firkin, J.-P. R. Hermet, P. O’Brien, J. Am. Chem. Soc. 2002, 124, 11870-11871.
[2] P. H. Lam, J. K. Kerkovius, S. E. Reisman, Org. Lett. 2023, 25, 8230-8233.
[3] J. D. Firth, S. J. Canipa, L. Ferris, P. O’Brien, Angew. Chem. Int. Ed. 2018, 57, 223-226.
