Home | 1‑Substituted isoquinolines synthesis by heterocyclization of TosMIC derivatives
1‑Substituted isoquinolines synthesis by heterocyclization of TosMIC derivatives
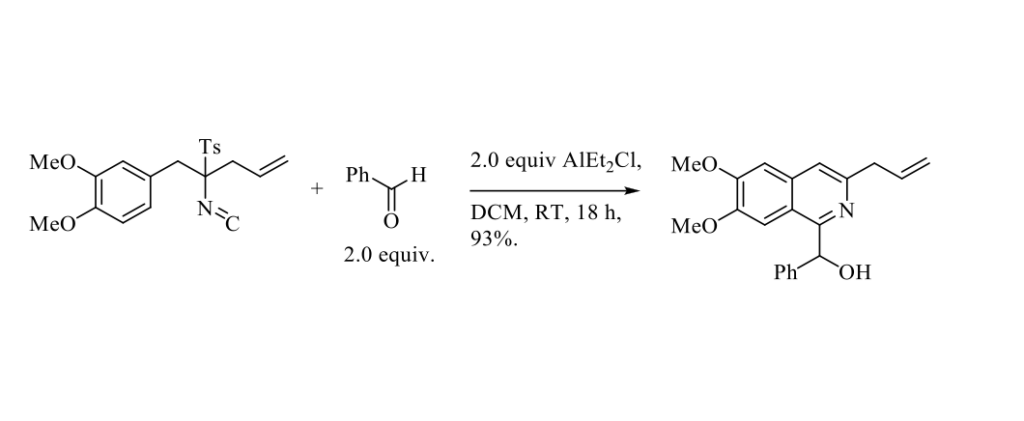
In 2016, Vaquero and Coll. published an original preparation of various 1‑substituted isoquinolines from monoalkylated α-benzylTosMIC derivatives and different electrophiles. [1] The later TosMIC derivatives were efficiently prepared from commercially available TosMIC reagent (toluenesulfonylmethyl isocyanide, also known as van Leusen’s reagent) in a single one-pot reaction under phase transfer conditions with NaOH in biphasic media (water and DCM) in the presence of TBAI (tetrabutylammonium iodide) with sequential addition of the two electrophiles. This heterocyclization of TosMIC derivatives is exemplified in Scheme below.
It should be pointed out that this heterocyclization is only efficient when electron-donating substituents are present on the benzene ring. A mechanism is proposed to explain this transformation. This heterocyclization has been successfully applied to an elegant total synthesis of Cassiarin A, an alkaloid isolated from Cassia siamea, displaying strong antimalarial activity.
[1] S. Gutiérrez, A. Coppola, D. Sucunza, C. Burgos, J. J. Vaquero, Org. Lett. 2016, 18, 3378-3381.
