Home | 1-Chloro-1,2-benziodoxol-3-one, an efficient electrophilic chlorinating reagent
1-Chloro-1,2-benziodoxol-3-one, an efficient electrophilic chlorinating reagent
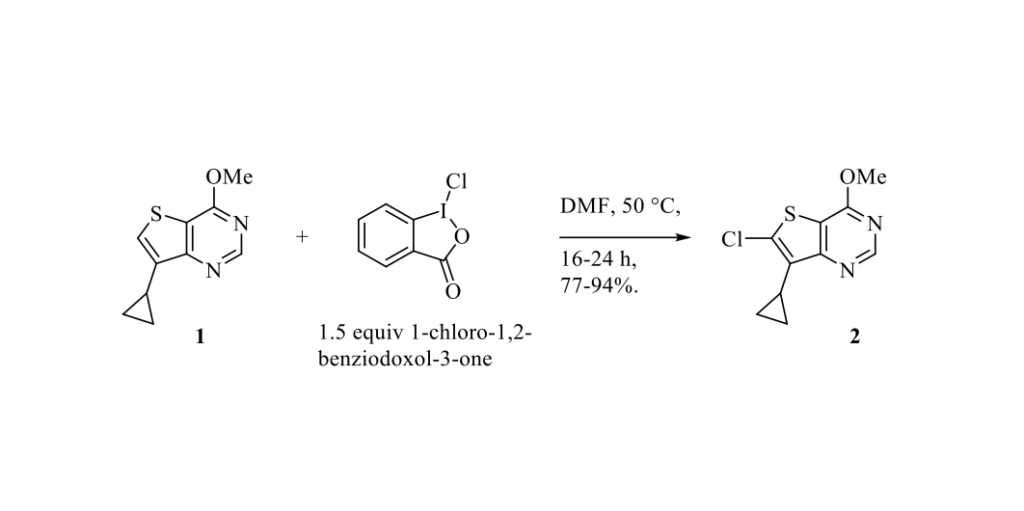
For the direct chlorination of arenes and heterocycles, various electrophilic reagents have been reported. In 2016, Xue and Coll. described the use of 1-chloro-1,2-benziodoxol-3-one, a hypervalent iodine(III) compound, as a mild and effective reagent for electrophilic chlorination. [1] In the synthesis of LP-922056 (an orally active, highly potent Notum Pectinacetylesterase inhibitor), treatment of thieno[3,2-d]pyrimidine derivative 1 (see Scheme below) with N-chlorosuccinimide (NCS) gave the desired chlorinated adduct 2 in low yields (around 15-32%) as well as by-products formed by ring opening of the cyclopropyl group. [2] In sharp contrast, the use of the air- and moisture-stable 1-chloro-1,2-benziodoxol-3-one provided the desired chlorinated intermediate 2 in 77-94% isolated yields. Only traces of cyclopropyl ring opening side-products were detected in the crude reaction mixture.
[1] M. Wang, Y. Zhang, T. Wang, C. Wang, D. Xue, J. Xiao, Org. Lett. 2016, 18, 1976-1979.
[2] N. J. Willis, E. D. Bayle, G. Papageorgiou, D. Steadman, B. N. Atkinson, W. Mahy, P. V. Fish, Beilstein J. Org. Chem. 2019, 15, 2790-2797.
