Home | Elegant access to quinazolines and pyrimidines by late-stage single-nitrogen atom insertion
Elegant access to quinazolines and pyrimidines by late-stage single-nitrogen atom insertion
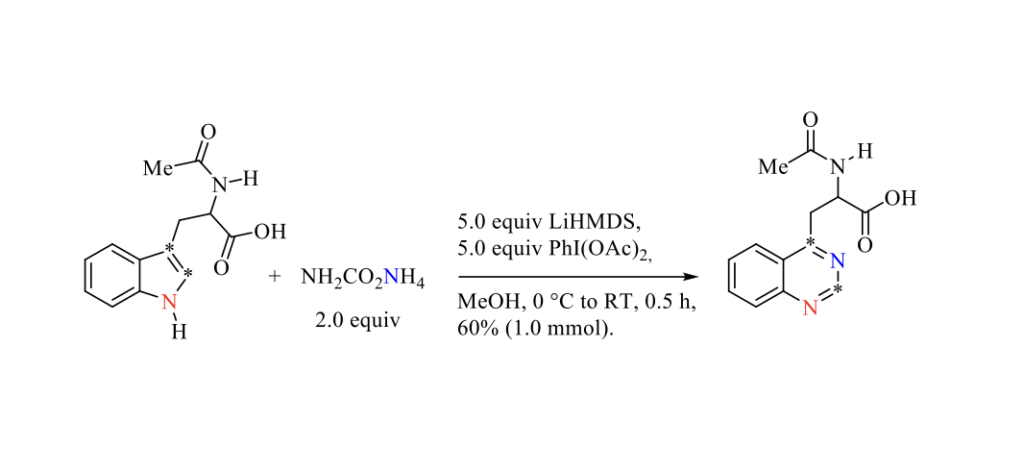
Late-stage nitrogen atom insertion into aromatic or heteroaromatic skeletons is an alternative strategy to prepare N-heterocyclic compounds compared to conventional ring construction strategies. [1] Very recently, Morandi and Coll. reported an elegant contribution in which a nitrogen atom is directly inserted into unprotected indoles and pyrroles to provide the corresponding quinazoline and pyrimidine derivatives, as highlighted in the Scheme below. [2]
A mechanism involving a [2+1] cycloaddition of an electrophilic iodonitrene species generated in situ to provide an aziridine intermediate which by elimination of iodobenzene followed by a concomitant ring expansion and aromatization is presented. [3]
[1] For recent references on this topic, see: (a) Y. He, J. Wang, T. Zhu, Z. Zheng, H. Wei, Chem. Sci. 2024, DOI: 10.1039/d3sc05367a; (b) P. Finkelstein, J. C. Reisenbauer, B. B. Botlik, O. Green, A. Florin, B. Morandi, Chem. Sci. 2023, 14, 2954-2959; (c) J. Wang, H. Lu, Y. He, C. Jing, H. Wei, J. Am. Chem. Soc. 2022, 144, 22433-22439.
[2] J. C. Reisenbauer, A.-Sophie K. Paschke, J. Krizic, B. B. Botlik, P. Finkelstein, B Morandi, Org. Lett. 2023, 25, 8419-8423.
[3] J. C. Reisenbauer, O. Green, A. Franchino, P. Finkelstein, B. Morandi, Science 2022, 377, 1104-1109.
