Home | Efficient preparation of 4-Cyanooxazoles from aldehydes
Efficient preparation of 4-Cyanooxazoles from aldehydes
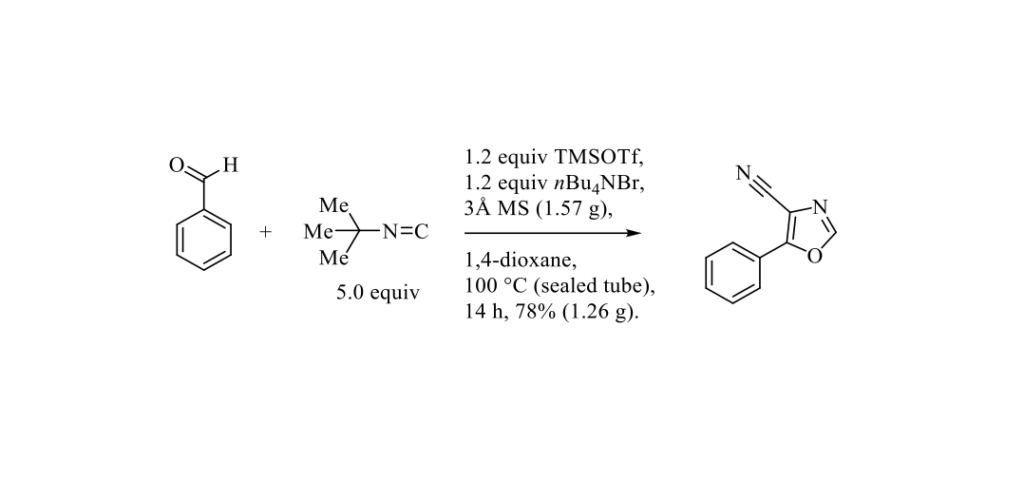
In medicinal chemistry, efficient methodologies to construct heterocycles on small scale from common functional groups are essential to generate molecular diversity. Very recently, B. Xu and Coll. published an efficient TMSOTf-promoted one-pot selective triple consecutive insertions of tert-butyl isocyanide into aldehydes to prepare 4-cyanooxazoles derivatives as outlined in the Scheme below. [1]
This methodology presents a wide substrates scope with around 50 examples. A mechanism is proposed for this reaction which involves three consecutive insertions of tert-butyl isocyanide.
[1] S. Lu, C.-H. Ding, B. Xu, Org. Lett. 2023, 25, 849-854.
