Home | An efficient multicomponent Mannich reaction to prepare substituted amines
An efficient multicomponent Mannich reaction to prepare substituted amines
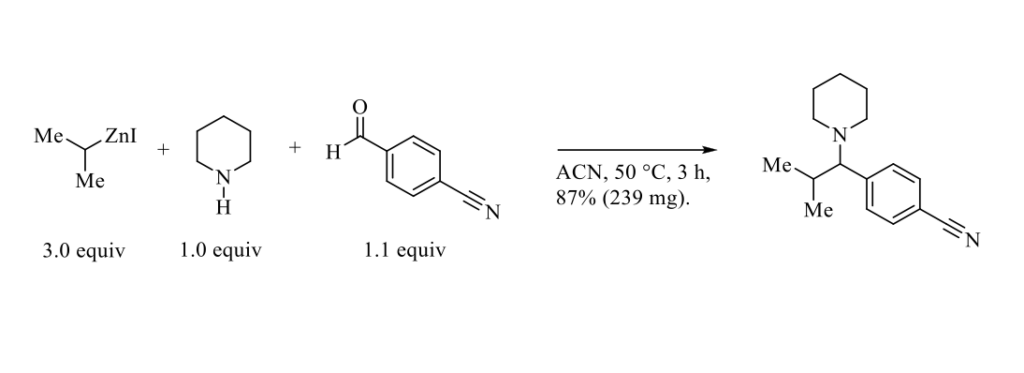
An efficient three-component one-pot condensation of primary, secondary, and tertiary organozinc reagents, secondary amines and aromatic or aliphatic aldehydes to afford a large variety of substituted amines (see Scheme below) has been very recently published by Le Gall, Presset et Coll. [1].
This simple protocol showing a broad scope of substrates, including aliphatic aldehydes, provides an excellent method to prepare densely substituted amines (37 examples). This Mannich-like three-component reaction presumably proceeds by the deprotonation of an hemiaminal intermediate (formed in situ) by the organozinc followed by the addition of a second equivalent of organozinc onto the resulting zinc alcoholate to afford the desired amine via a putative 6-membered transition state. [2]
[1] M. Pinaud, E. Le Gall, M. Presset, J. Org. Chem. 2022, 87, 4961-4964.
[2] E. Le Gall et al., J. Organomet. Chem. 2013, 736, 27-35.
