Home | An efficient and mild preparation of terminal alkynes from alkenyl triflates
An efficient and mild preparation of terminal alkynes from alkenyl triflates
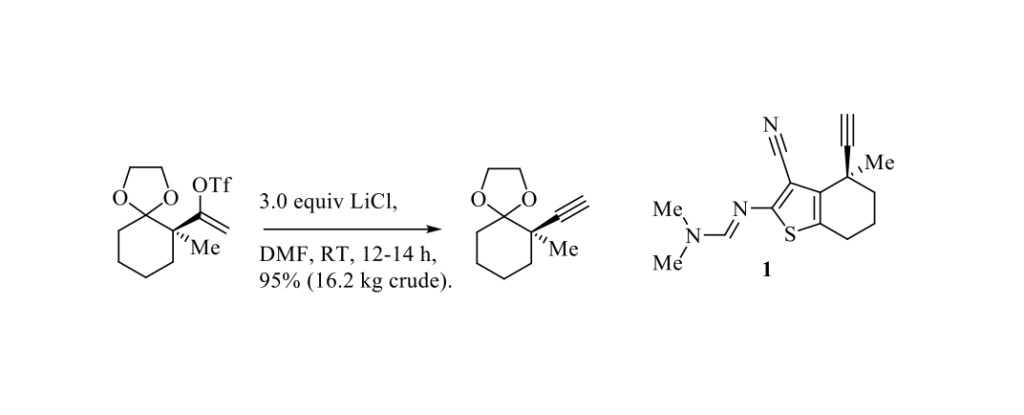
As part of the development of a kilogram-scale route to KRAS G12C inhibitors for the treatment of cancer, J. C. Leung and Coll. from Boehringer Ingelheim Pharmaceuticals Inc. (Ridgefield, Connecticut, USA) have published a preparation of the key building block 1 (see Scheme below). [1] To form the terminal alkyne, the vinyl triflate was treated with an excess of LiCl in DMF at room temperature to afford the desired adduct in 95% crude yield on 16.2 kg scale. [2] The use of LiCl proved to be more efficient than strong bases such as LDA or LiHMDS. It should be pointed out that to promote the formation of terminal alkynes from vinyl triflates, pyridine [3] or TBAF [4] have been also used instead of LiCl.
[1] J. C. Leung et al., Org. Process Res. Dev. 2024, 28, 67-77.
[2] X. Yang, D. Wu, Z. Lu, H. Sun, A. Li, Org. Biomol. Chem. 2016, 14, 5591-5594.
[3] J. Ramharter, H. Weinstabl, J. Mulzer, J. Am. Chem. Soc. 2010, 132, 14338-14339.
[4] M. Okutani, Y. Mori, Chem. Pharm. Bull. 2015, 63, 393-396.
