Home | A large-scale Shi asymmetric epoxidation
A large-scale Shi asymmetric epoxidation
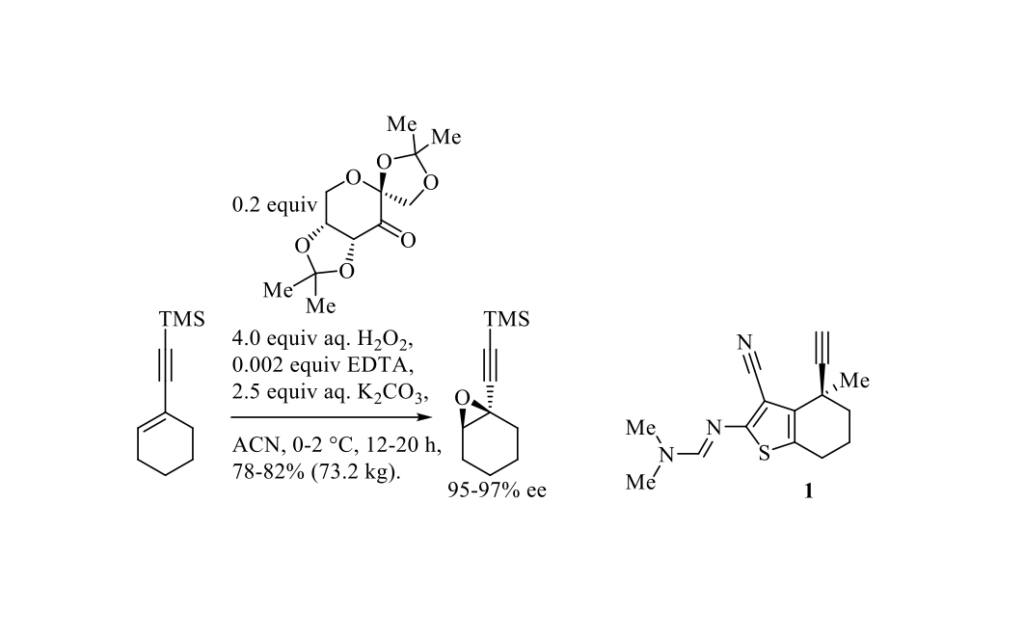
The asymmetric epoxidation of alkenes with oxone® (2KHSO5·KHSO4·K2SO4) and a fructose-derived ketone (epoxone) catalyst is known as Shi epoxidation. [1] This highly enantioselective epoxidation involves a dioxirane intermediate, generated from the ketone catalyst by oxone®. Very recently, J. J. Song and Coll. from Boehringer Ingelheim Pharmaceuticals (Ridgefield, Connecticut, United States) described a large-scale asymmetric synthesis of KRAS G12C inhibitor building block 1 in which the quaternary stereocenter was installed enantioselectively by Shi epoxidation, as outlined in Scheme below. [2]
The D-epoxone used in this protocol is easily prepared in two steps from inexpensive D-fructose, while the opposite enantiomer is obtained from readily available L-sorbose, following a five-step synthetic route.
[1] Z.-X. Wang, Y. Tu, M. Frohn, J.-R. Zhang, Y. Shi, J. Am. Chem. Soc. 1997, 119, 11224-11235.
[2] Z. Tan, J. J. Song et al., Org. Process Res. Dev. 2024, 28, 78-91.
